this post was submitted on 24 Jul 2024
648 points (98.4% liked)
Science Memes
11189 readers
3050 users here now
Welcome to c/science_memes @ Mander.xyz!
A place for majestic STEMLORD peacocking, as well as memes about the realities of working in a lab.

Rules
- Don't throw mud. Behave like an intellectual and remember the human.
- Keep it rooted (on topic).
- No spam.
- Infographics welcome, get schooled.
This is a science community. We use the Dawkins definition of meme.
Research Committee
Other Mander Communities
Science and Research
Biology and Life Sciences
- !abiogenesis@mander.xyz
- !animal-behavior@mander.xyz
- !anthropology@mander.xyz
- !arachnology@mander.xyz
- !balconygardening@slrpnk.net
- !biodiversity@mander.xyz
- !biology@mander.xyz
- !biophysics@mander.xyz
- !botany@mander.xyz
- !ecology@mander.xyz
- !entomology@mander.xyz
- !fermentation@mander.xyz
- !herpetology@mander.xyz
- !houseplants@mander.xyz
- !medicine@mander.xyz
- !microscopy@mander.xyz
- !mycology@mander.xyz
- !nudibranchs@mander.xyz
- !nutrition@mander.xyz
- !palaeoecology@mander.xyz
- !palaeontology@mander.xyz
- !photosynthesis@mander.xyz
- !plantid@mander.xyz
- !plants@mander.xyz
- !reptiles and amphibians@mander.xyz
Physical Sciences
- !astronomy@mander.xyz
- !chemistry@mander.xyz
- !earthscience@mander.xyz
- !geography@mander.xyz
- !geospatial@mander.xyz
- !nuclear@mander.xyz
- !physics@mander.xyz
- !quantum-computing@mander.xyz
- !spectroscopy@mander.xyz
Humanities and Social Sciences
Practical and Applied Sciences
- !exercise-and sports-science@mander.xyz
- !gardening@mander.xyz
- !self sufficiency@mander.xyz
- !soilscience@slrpnk.net
- !terrariums@mander.xyz
- !timelapse@mander.xyz
Memes
Miscellaneous
founded 2 years ago
MODERATORS
you are viewing a single comment's thread
view the rest of the comments
view the rest of the comments
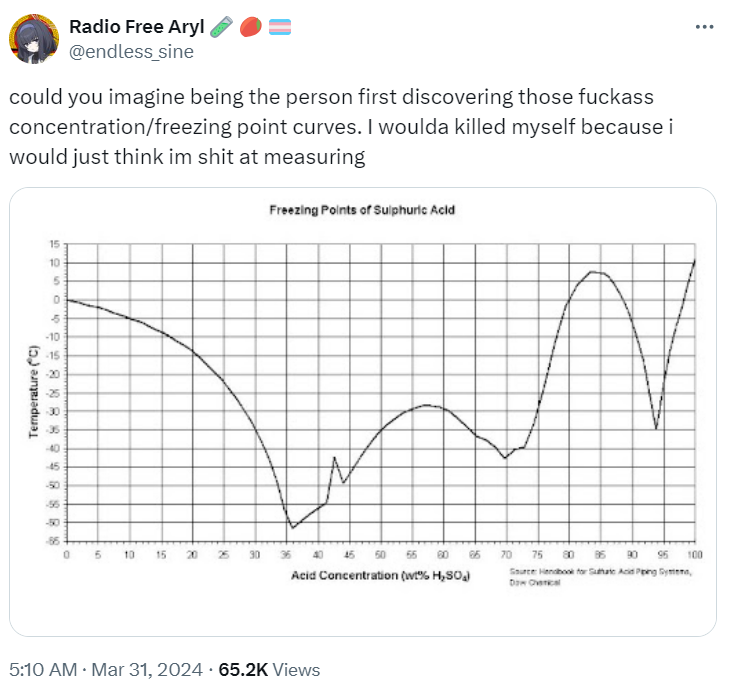
Dare someone smarter than me explain what the ever loving fuck is going on there?
This is what happens when some kind of new compound is formed between these two, here it'll be series of sulfuric acid-water complexes. Same thing happens with metals when intermetallic compounds form, see titanium-nickel phase diagram. Normal case would be eutectic, see aluminum-silicon phase diagram
Here is a more complete chart https://chemistry.stackexchange.com/questions/165279/details-of-what-actually-happens-in-cold-sulfuric-acid-between-80-and-90-what
I see. So these are actually many sulfuric compounds in a trenchcoat chart.
Some of which are probably only a bit stable and so only exist in a mixture
Never mind between 80 and 90%, WTF is happening at 42.5%‽
There's a metastable phase somewhere out there and change of the most stable solid phase between 4-hydrate and 6.5-hydrate
My uneducated understanding is that the chart shows at which temperatures sulfuric acid freezes depending on the concentration. Also in my very basic understanding of physics and chemistry I would have thought that it's linear or exponential or something predictable and not that jumpy.
In normal cases you'd see two curves going away from pure compounds downwards to a common minimum, which is eutectic point. It's generally only vaguely predictable, but always monotonic
Here's what a normal curve looks like:
https://i2.wp.com/www.storksplows.com/docs/wp-content/uploads/2017/04/Brine-Pro-salinity-chart.png
Sulfuric acid and water has various H2SO4 and H2O ratios. So like 1 H2SO4 and 6, 3, 2, or 1 H2O it also has just the H2SO4 and H2S2O7. These are present as local points within solutions and with different prominence depending on the amount of water added. These 8 different ratios each have different freezing points.
If I had to guess, I would assume that there are different molecular lattices that sulfuric acid and water can form at different concentrations and that these different lattices have different freezing points. I will now go look it up.
What you're describing are different crystalline phases of pure compounds, but this does not give you new minima, you need some new compound to form for that
Physics.